報(bào)告題目:Crystal Engineering Through Batch and Continuous Process
報(bào)告時間:2015年12月29日9:00 AM
報(bào)告地點(diǎn):beat365245會議室
報(bào)告人:DrGuangyang Hou
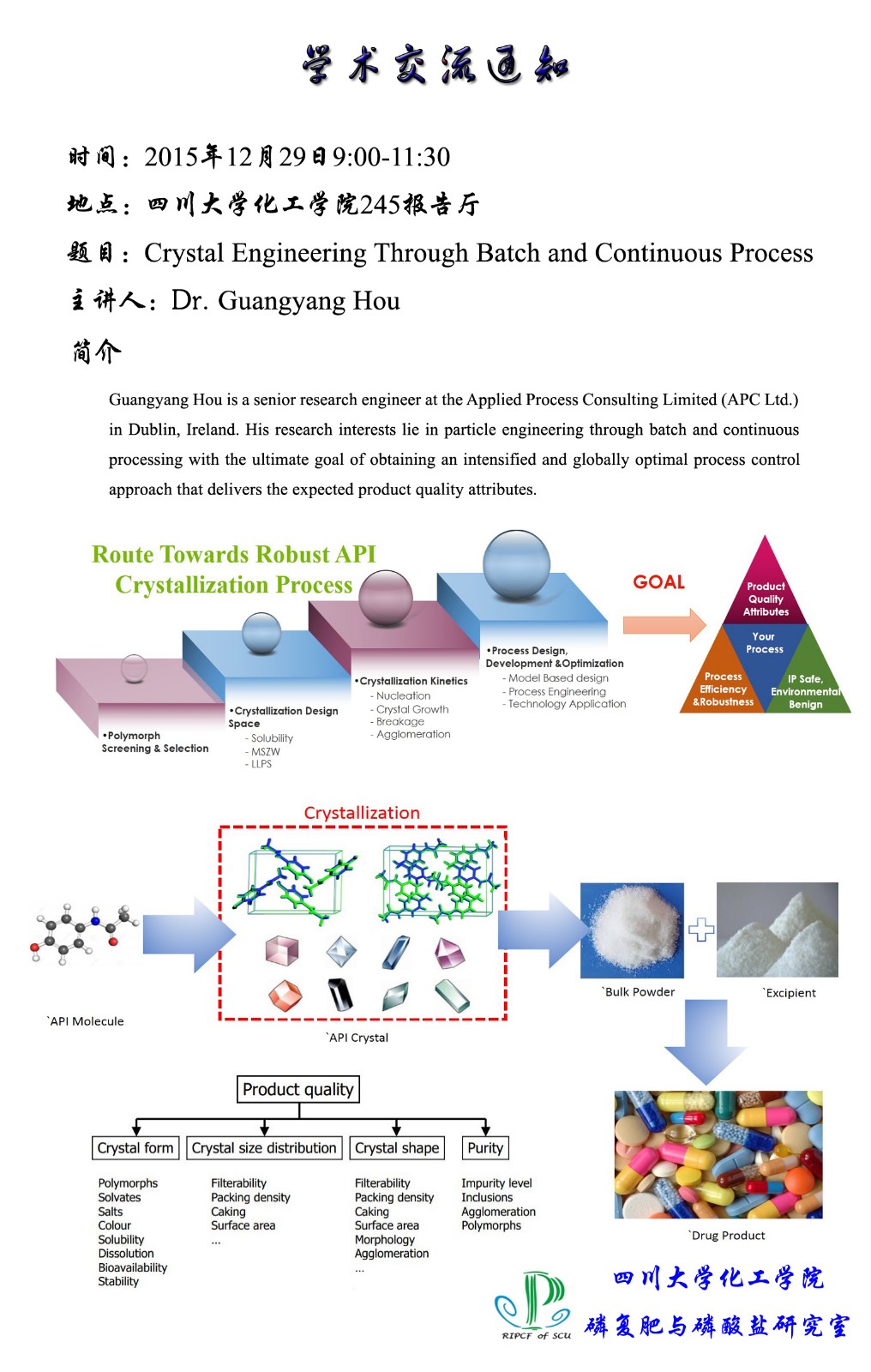
摘 要:Crystallization is an essential separation and purification operation in the pharmaceutical industry as over 90 % of active pharmaceutical ingredients are crystals of small organic molecules. Along with the United States Food and Drug Administration’s (FDA) Process Analytical Technology (PAT) initiative, the development of control approaches, which can improve the manufacturing of products with desired properties such as crystal size distribution (CSD), habit and polymorphic form, has become of significant important. In this presentation, model based free approaches including supersaturation and direct nucleation control via batch mode and continuous processing via plug-flow reactor and the mixed product, mixed-suspension removal (MSMPR) crystallizers to engineer particle attributes will be detailed.