Recently, the group of Dawen Niu published a paper entitled “Asymmetric O-propargylation of secondary aliphatic alcohols” inNature Catalysis.
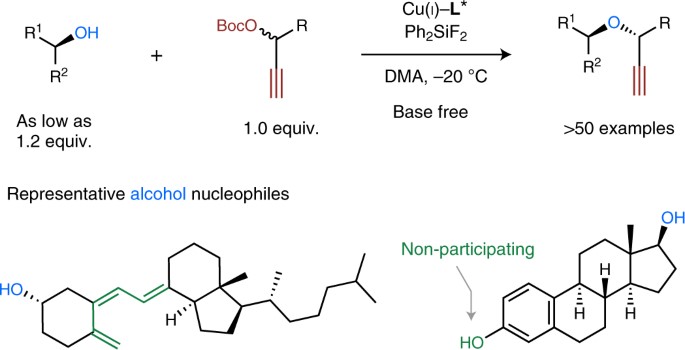
The asymmetric O-alkylation of secondary aliphatic alcohols remains a substantial challenge in chemistry. Such a challenge largely stems from the steric demand of each reactant, in addition to the relatively low nucleophilicity of alcohols. Here, we report the development of a base-free, Cu-catalysed propargylic substitution reaction that enables the efficient, asymmetric O-propargylation of secondary aliphatic alcohols. Mechanistic studies implied key factors to slow down the undesired decomposition process of electrophiles in this reaction, which opened up the possibility of using secondary aliphatic alcohols as nucleophilic substrates. This asymmetric O-alkylation reaction proceeds under almost neutral conditions, tolerates a broad scope of functional groups and shows remarkable chemoselectivities. This method is amenable to the modification of natural products and commercial drugs. The products obtained could be readily elaborated to various classes of enantioenriched α,α′-disubstituted ethers that are difficult to access by other methods.
For link of this paper: https://www.nature.com/articles/s41929-020-0462-9.
