
In collaboration with the Houk’s team from the University of California, Los Angeles, Professor Dawen Niu’s team of the State Key Laboratory of Biotherapy and Cancer Center of West China Hospital and School of Chemical Engineering published their research findings entitled "Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation" inNature Chemistryon April 11, 2022. SCU is the first work unit of the thesis, and Dawen Niu is the co corresponding author.
Sugars play an indispensable role in almost all physiological and pathological processes, such as immunity, bacterial and viral infection, tumorigenesis and migration. Almost all major human diseases are related to sugars. At present, more than 100 small molecule drugs on the market are carbohydrate drugs; and many active natural products also contain glycosyl side chains. In-depth study of carbohydrate compounds is an important platform for understanding life processes and developing new drugs as well. Therefore, it is of great significance to develop simple, efficient and highly selective glycosidic bonds.
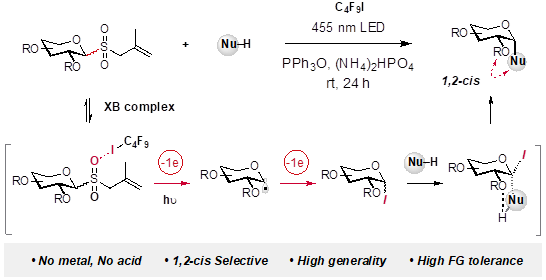
Radical activation andmechanistic explanation for 1,2-cisselectivity.
“--- Here we report a strategy for 1,2-cis-glycosylation without using metals, strong (Lewis) acids, elaborate catalysts or labile substrates. Our method operates by a unique mechanism: it activates glycosyl donors through a radical cascade rather than the conventional acid-promoted, ionic process. As elucidated by computational and experimental studies, the allyl glycosyl sulfones (as donors) form halogen bond complexes with perfluoroalkyl iodides, which—merely by visible light irradiation—fragment via radical intermediates to give the electrophilic glycosyl iodides. In situ trapping by various nucleophiles affords, in a stereoconvergent manner, the challenging 1,2-cis-glycosides. This metal- and acid-free reaction shows remarkable tolerance to functional groups. The high stereoselectivity holds for a broad array of donors. This study suggests that the simple C2-alkoxy group can serve as an effective directing group for building 1,2-cis-glycosidic bonds.”(Abstract)
The research work is supported by funding from the National Natural Science Foundation of China (nos. 21922106 and 21772125) and the 1.3.5 Project for Disciplines of Excellence, West China Hospital. Additional support (K.N.H.) came from the National Science Foundation of the United States (CHE-1764328). We thank X. Wang from the Analytical and Testing Center of Sichuan University for NMR experiments.
https://www.nature.com/articles/s41557-022-00918-z
